- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
With reference to the report published, the global non-invasive prenatal testing (NIPT) market size was prized by USD 2.83 billion in 2020. It is estimated to witness a 10.9% CAGR from 2021 to 2028.
The growing occurrence of chromosomal abnormalities together with the increasing use of the product in novel functions is pouring the natural revenue enlargement within the market. Besides, progressions in the presented tests, in terms of enhanced chemistry, bioinformatics study, and sophisticated function are the factors, expected to stimulate the enlargement of the market for non-invasive prenatal testing.
Enhancing procedures of reimbursement, for the low-risk as well as standard pregnancy, is one of the major encouraging factors of the market. Natera declared extended treatment from the leading U.S. Health Plan, Aetna, for its non-invasive prenatal testing to each and everyone pregnancy, in December 2020.
The severe necessity to monitor for and test huge numbers of patients for probable Sars-Cov-2 disease is the main universal concern of every nation’s government, affected by the Covid-19 disease. Consequently, the majority of them are in front of the major shortage in the quantity, for diagnostic kits to assessment for the virus. Virology diagnostics units are under huge pressure to offer dependable testing kits, and there is a gush in demand for point-of-care or in-vitro testing facilities by labs in many nations.
On account of the greater acceptance of NGS-based prenatal tests, which involves a variety of reagents & assays to operate sequencing procedures, consumables & reagents retained the biggest revenue share in the non-invasive prenatal testing market, in 2020 and it was more than 76%. The existence of a sizeable amount of suppliers, which presents solutions to segregate cell-free DNA, is moreover inspiring the enlargement of the section.
As a result of incessant hard work carried out by the instrument manufacturing companies to improve sophisticated platforms, the instruments division is anticipated to record the highest CAGR, throughout the forecast period.
NGS technology held the biggest, above 57% share in 2020, and dominated the non-invasive prenatal testing (NIPT) market. This is due to the extensive use of NGS technology, together with whole-exome sequencing, targeted genome sequencing, and whole-genome sequencing, intended for prenatal cfDNA tests. Whole-genome sequencing puts forward confirmed advantages above the new technologies and contains a relatively lesser failure percentage.
The NGS technology is leading in each and every one feature together with penetration, efficiency, technology as well as availability. This has caused the extensive use of NGS, in this area. Moreover, as compared to usual karyotyping, the superior effectiveness of Chromosomal Microarray Analysis (CMA) increases revenue, within this section.
The cell-free DNA-based NIPT market was expected to produce the maximum 43.6% revenue share, in 2020. Cell-free DNA is, gradually more, employed in forecasting the hazard of the genetic situation in prenatal care, in the course of diverse genetic investigations. Numerous research analyses are done to; additionally increase the usage of the tests, presented over this segment.
Ultrasound detection is arranged as balancing to the Cell-free DNA-based non-invasive prenatal testing, causing the lesser share of this section, in the present market.
The 13 to 24 weeks gestation period section held above 45% revenue share, in 2020 and dominated the market. This can be credited to the information, that most non-invasive prenatal analysis is performed in the subsequent trimester of the pregnancy. These examinations, furthermore, deal with the key complementary checks, such as serum and ultrasound screening for the alpha-fetoprotein.
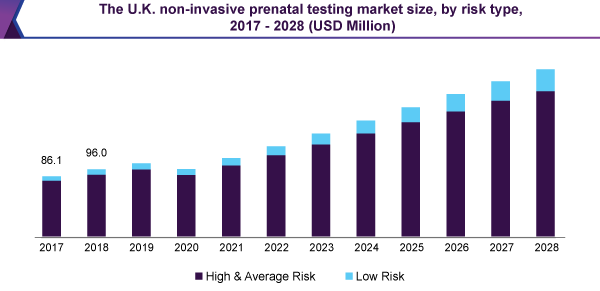
Owing to the extreme adoption of these examinations in high-risk cases, for the patients having an age group of 35 years and more, the market for non-invasive prenatal testing for high and standard risk pregnancies, detained the biggest, more than 90% share in 2020.
The existence of encouraging customer repayment policy, in this section, together with increasing alertness regarding the avoidance of chromosomal anomalies, like down syndrome, has furthermore added to the bigger share of this section. In contrast, the low-risk pregnancy test market is expected to observe the highest CAGR, during the forecast period.
Backing from the government, similar to the assignment of finances for the average-risk pregnancies, is estimated to be the positive thing for the expansion of the market.
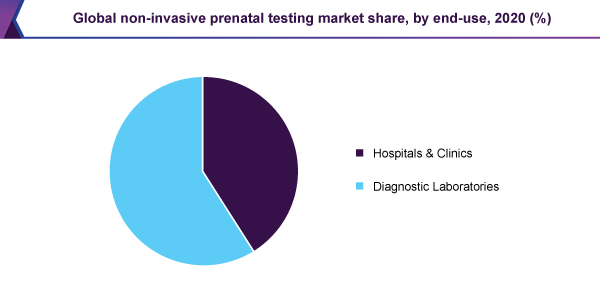
Diagnostic laboratories held a 59% revenue share in 2020 and dominated the market. At a time when the internal amenities are not adequate, an important developer like Illumina is contracting out their sample processing to Illumina CLIA labs. Moreover, the laboratories, that are busy performing non-invasive prenatal testing through the U.S., are working with the quality assurance system, to maintain the reproducibility and quality of the test.
North America retained the highest more than 45.9%, share in 2020. It was tracked by Europe. The U.S. requires nationwide agreement strategies on the execution of non-invasive prenatal testing; yet, it is extensively accepted through the nation, causing the domination of North America. Besides, the new adding up of non-invasive prenatal testing in the treatment policy of main insurance companies is expected to increase the enlargement of the market, within the U.S.
The U.K. High Court gave the decision affirming Harmony cell-free, DNA sourced non-invasive prenatal testing. It was developed by Ariosa. It breaches the European copyright, released by Illumina. The decision of the court guarantees the legality of patent entitlement. Such types of actions are pushing revenue enlargement within the region.
The Asia Pacific is expected to be the rapidly developing local market, throughout the forecast period. The existence of the companies, for example, BGI in China, which is a major entity in the market for non-invasive prenatal testing, is projected to increase the market expansion.
The companies, busy in the improvement of non-invasive prenatal testing solutions, are working in extremely aggressive business backgrounds. Main companies are increasing their test range, to differentiate themselves and set their share in the market. The companies are, moreover, concentrating on incessant modernization as well as presenting products, to obtain and maintain the higher shares of the market. Likewise, together with increasing the utilization of present products, the companies are developing the products, as per the international requirement.
• Quest Diagnostics, Inc.
• Qiagen
• Myriad Women’s Health, Inc. (Myriad Genetics Inc/Counsyl, Inc.)
• Med Genome Labs Ltd.
• Illumina, Inc. (Verinata Health, Inc.)
• Natera, Inc.
• Laboratory Corp. of America Holdings
• Progenity, Inc.
• F. Hoffmann-La Roche Ltd. (Ariosa Diagnostics)
• Euro fins LifeCodexx GmbH
• Cento gene N.V.
• Genesis Genetics (Cooper Surgical, Inc.)
|
Report Attribute |
Details |
|
The market size value in 2021 |
USD 3.25 billion |
|
The revenue forecast in 2028 |
USD 6.47 billion |
|
Growth Rate |
CAGR of 10.9% from 2021 to 2028 |
|
The base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Gestation period, risk type, method, technology, product, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Japan; China; India; Singapore; Australia; Brazil; Mexico; South Africa; Saudi Arabia |
|
Key companies profiled |
Genesis Genetics (CooperSurgical, Inc.); Natera, Inc.; Centogene N.V.; Illumina, Inc. (Verinata Health, Inc.); Eurofins LifeCodexx GmbH; MedGenome Labs Ltd.; F. Hoffmann-La Roche Ltd. (Ariosa Diagnostics); Myriad Women’s Health, Inc. (Myriad Genetics Inc/Counsyl, Inc.); Progenity, Inc.; Qiagen; Laboratory Corp. of America Holdings; Quest Diagnostics, Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2017 to 2028. For the purpose of this study, Million Insight's has segmented the global non-invasive prenatal testing market report on the basis of the gestation period, risk type, method, technology, product, end-use, and region:
• Gestation Period Outlook (Revenue, USD Million, 2017 - 2028)
• 0-12 Weeks
• 13-24 Weeks
• 25-36 Weeks
• Risk Type Outlook (Revenue, USD Million, 2017 - 2028)
• High & Average Risk
• Low Risk
• Method Outlook (Revenue, USD Million, 2017 - 2028)
• Ultrasound Detection
• Biochemical Screening Tests
• Cell-Free DNA in Maternal Plasma Tests
• Technology Outlook (Revenue, USD Million, 2017 - 2028)
• NGS
• Array Technology
• PCR
• Other Technologies
• Product Outlook (Revenue, USD Million, 2017 - 2028)
• Consumables & Reagents
• Instruments
• End-use Outlook (Revenue, USD Million, 2017 - 2028)
• Hospitals & Clinics
• Diagnostic Laboratories
• Regional Outlook (Revenue, USD Million, 2017 - 2028)
• North America
• U.S.
• Canada
• Europe
• Germany
• U.K.
• France
• Italy
• Spain
• The Asia Pacific
• Japan
• China
• India
• Singapore
• Australia
• Latin America
• Brazil
• Mexico
• MEA
• South Africa
• Saudi Arabia


Research Support Specialist, USA